Quiz Summary
0 of 40 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 40 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 40
1. Question
Title: Human Papillomavirus DNA versus Papanicolaou Screening Tests for Cervical Cancer
Objective:To determine whether oncogenic human papillomavirus (HPV) DNA testing is superior to the Papanicolaou (Pap) test for cervical cancer screening.
Methods:
Design: Randomized clinical trial
Blinding: Double-blinded
Setting: 30 clinics in Montreal and St. John’s, Canada.
Patients: Women ages 30–69 who sought screening tests for cervical cancer. Patients who were currently being followed-up for a cervical lesion, lacked a cervix, were pregnant, had a history of cervical cancer, had undergone Pap testing in the previous year, or were unable to provide consent were excluded.
Intervention: Patients were randomized in a 1:1 ratio to a “Pap focus” or an “HPV focus” screening group. Both tests were administered sequentially during the same visit. However, in the “Pap focus” group, participants received the Pap test first, whereas in the “HPV focus” group, patients received the HPV test first. Study participants with abnormal Pap test results or a positive HPV test (at least 1 pg of high-risk HPV DNA per mL) underwent colposcopy and biopsy, as did a random sample of women with negative tests.
Outcome measures: Ability of Pap and HPV DNA testing to detect grade 2 or 3 cervical intraepithelial neoplasia, adenocarcinoma in situ, or cervical cancers (confirmed histologically and by loop electrosurgical excision procedure).
Results:A total of 10,154 women were randomly assigned to testing groups. Both tests were performed on all women in a randomly assigned sequence during the same visit. The sensitivities and specificities of the 2 tests, alone and in combination, are shown in the table below.
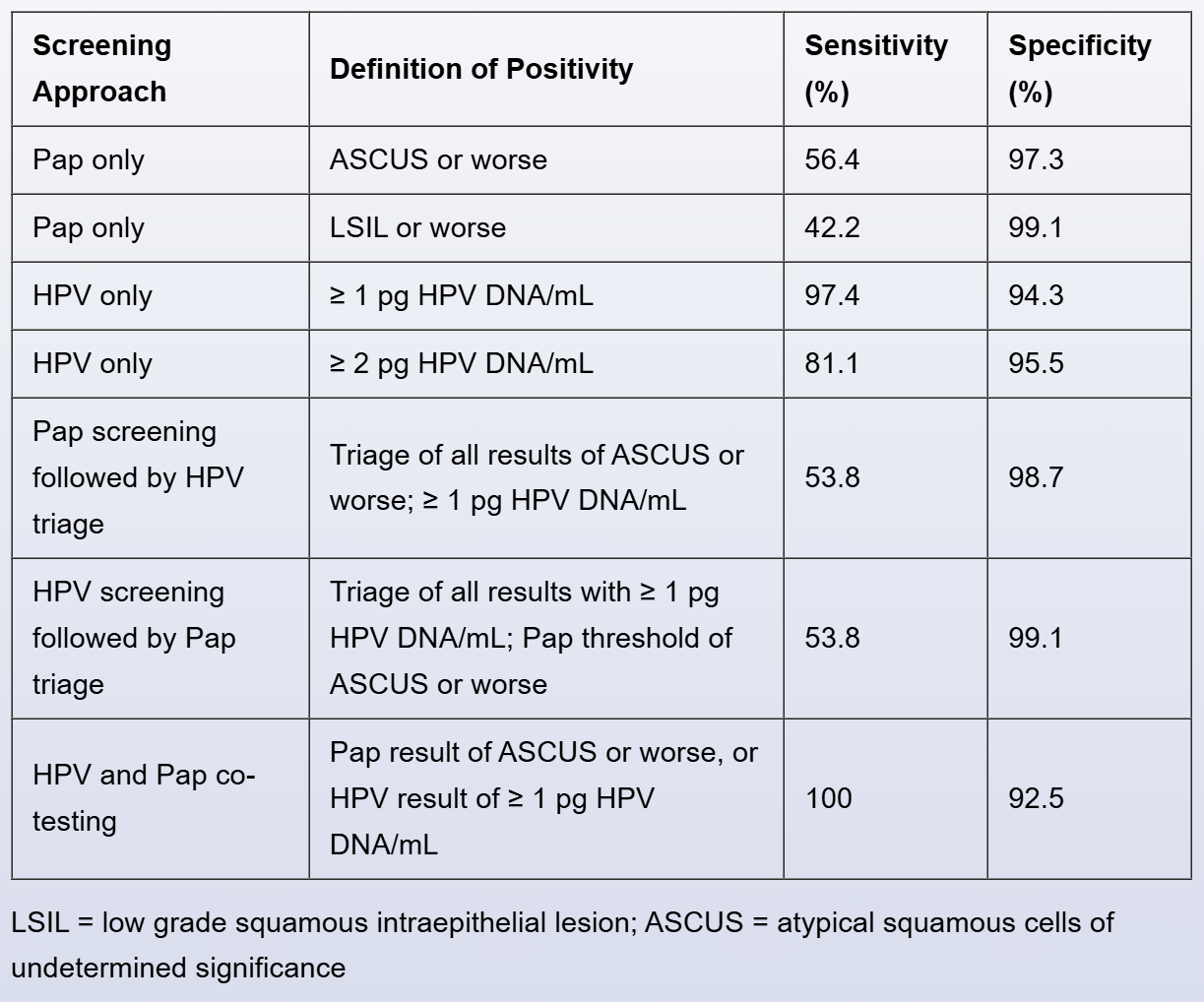
Conclusion: Compared to Pap testing, HPV DNA testing has greater sensitivity for the detection of cervical intraepithelial neoplasia.
Funding Source: Supported by a grant (MCT-54063) from the Canadian Institutes of Health Research and partially by an unrestricted grant from Merck Frosst Canada.
Structured abstract is based on: NEJM 2007;357(16): 1579-1588.
The abstract shown above applies to the next 3 items
A 33-year-old woman comes to the clinic for a routine health maintenance examination. She has no lower abdominal pain, vaginal bleeding, or vaginal discharge. Her past medical history is remarkable for 1 episode of uncomplicated urinary tract infection treated with a short course of antibiotics. She is currently not taking any medications. She has had 5 sexual partners in her life and has used barrier contraception the majority of the time. Her latest sexual relationship began 3 months ago. Her last cervical cancer screening examination was a Pap smear performed at the age of 23 that showed no abnormalities. She does not use tobacco, alcohol, or illicit drugs. During her visit, she requests screening for cervical cancer and appears extremely concerned as her older sister was recently diagnosed with it.
Item 1 of 3
Based on the study results, which of the following testing sequences would be most effective for ruling out cervical neoplasia in this patient?
CorrectIncorrect -
Question 2 of 40
2. Question
Title: Human Papillomavirus DNA versus Papanicolaou Screening Tests for Cervical Cancer
Objective:To determine whether oncogenic human papillomavirus (HPV) DNA testing is superior to the Papanicolaou (Pap) test for cervical cancer screening.
Methods:
Design: Randomized clinical trial
Blinding: Double-blinded
Setting: 30 clinics in Montreal and St. John’s, Canada.
Patients: Women ages 30–69 who sought screening tests for cervical cancer. Patients who were currently being followed-up for a cervical lesion, lacked a cervix, were pregnant, had a history of cervical cancer, had undergone Pap testing in the previous year, or were unable to provide consent were excluded.
Intervention: Patients were randomized in a 1:1 ratio to a “Pap focus” or an “HPV focus” screening group. Both tests were administered sequentially during the same visit. However, in the “Pap focus” group, participants received the Pap test first, whereas in the “HPV focus” group, patients received the HPV test first. Study participants with abnormal Pap test results or a positive HPV test (at least 1 pg of high-risk HPV DNA per mL) underwent colposcopy and biopsy, as did a random sample of women with negative tests.
Outcome measures: Ability of Pap and HPV DNA testing to detect grade 2 or 3 cervical intraepithelial neoplasia, adenocarcinoma in situ, or cervical cancers (confirmed histologically and by loop electrosurgical excision procedure).
Results:A total of 10,154 women were randomly assigned to testing groups. Both tests were performed on all women in a randomly assigned sequence during the same visit. The sensitivities and specificities of the 2 tests, alone and in combination, are shown in the table below.

Conclusion: Compared to Pap testing, HPV DNA testing has greater sensitivity for the detection of cervical intraepithelial neoplasia.
Funding Source: Supported by a grant (MCT-54063) from the Canadian Institutes of Health Research and partially by an unrestricted grant from Merck Frosst Canada.
Structured abstract is based on: NEJM 2007;357(16): 1579-1588.
Item 2 of 3
A national government agency is reviewing data from this paper to decide which screening method to recommend and fund. However, agency officials realize that the prevalence of cervical cancer varies throughout the country. For this reason, a reliable epidemiological parameter is needed to compare the significance of negative and positive results obtained in individual patients, irrespective of prevalence. Which of the following epidemiological parameters would be the most useful in comparing these screening tests when applied to individual patients?
CorrectIncorrect -
Question 3 of 40
3. Question
Title: Human Papillomavirus DNA versus Papanicolaou Screening Tests for Cervical Cancer
Objective:To determine whether oncogenic human papillomavirus (HPV) DNA testing is superior to the Papanicolaou (Pap) test for cervical cancer screening.
Methods:
Design: Randomized clinical trial
Blinding: Double-blinded
Setting: 30 clinics in Montreal and St. John’s, Canada.
Patients: Women ages 30–69 who sought screening tests for cervical cancer. Patients who were currently being followed-up for a cervical lesion, lacked a cervix, were pregnant, had a history of cervical cancer, had undergone Pap testing in the previous year, or were unable to provide consent were excluded.
Intervention: Patients were randomized in a 1:1 ratio to a “Pap focus” or an “HPV focus” screening group. Both tests were administered sequentially during the same visit. However, in the “Pap focus” group, participants received the Pap test first, whereas in the “HPV focus” group, patients received the HPV test first. Study participants with abnormal Pap test results or a positive HPV test (at least 1 pg of high-risk HPV DNA per mL) underwent colposcopy and biopsy, as did a random sample of women with negative tests.
Outcome measures: Ability of Pap and HPV DNA testing to detect grade 2 or 3 cervical intraepithelial neoplasia, adenocarcinoma in situ, or cervical cancers (confirmed histologically and by loop electrosurgical excision procedure).
Results:A total of 10,154 women were randomly assigned to testing groups. Both tests were performed on all women in a randomly assigned sequence during the same visit. The sensitivities and specificities of the 2 tests, alone and in combination, are shown in the table below.

Conclusion: Compared to Pap testing, HPV DNA testing has greater sensitivity for the detection of cervical intraepithelial neoplasia.
Funding Source: Supported by a grant (MCT-54063) from the Canadian Institutes of Health Research and partially by an unrestricted grant from Merck Frosst Canada.
Structured abstract is based on: NEJM 2007;357(16): 1579-1588.
Item 3 of 3
During the study, women with abnormal Pap test results or a positive HPV test underwent colposcopy and biopsy, as did a random sample of women with negative test results. The purpose of performing a colposcopy and a biopsy on women with negative test results is to reduce which type of bias?
CorrectIncorrect -
Question 4 of 40
4. Question
Hypothesis: Are higher glucose levels associated with increased risk of dementia?
Methods:
Design: Cohort study.
Setting: Randomly selected members of a single health care system.
Follow-up: Median follow-up of 6.7 years.
Patients: 2,075 participants age ≥65, with diabetes (N = 230) and without diabetes (N = 1,845), all without dementia at baseline.
Exposure variable: Average glucose level for each participant at baseline and in 5-year rolling windows (based on 34,904 clinical measurements of glucose levels and/or 10,518 measurements of glycated hemoglobin levels, with standard transformations according to predetermined formulae applied to arrive at average level for each participant).
Outcome measure: Diagnosis of dementia based on the initial screening with Cognitive Abilities Screening Instrument and confirmed by further evaluation.
Data analysis: Cox regression models stratified according to diabetes status and adjusted for age, sex, study cohort, educational level, level of exercise, blood pressure, and status with respect to coronary and cerebrovascular diseases, atrial fibrillation, smoking, and treatment for hypertension.
Results: During follow-up, dementia developed in 540 participants (79 with diabetes and 461 without). The table reports the adjusted risk of incident dementia associated with the average glucose level over the preceding 5 years among participants without diabetes and those with diabetes.
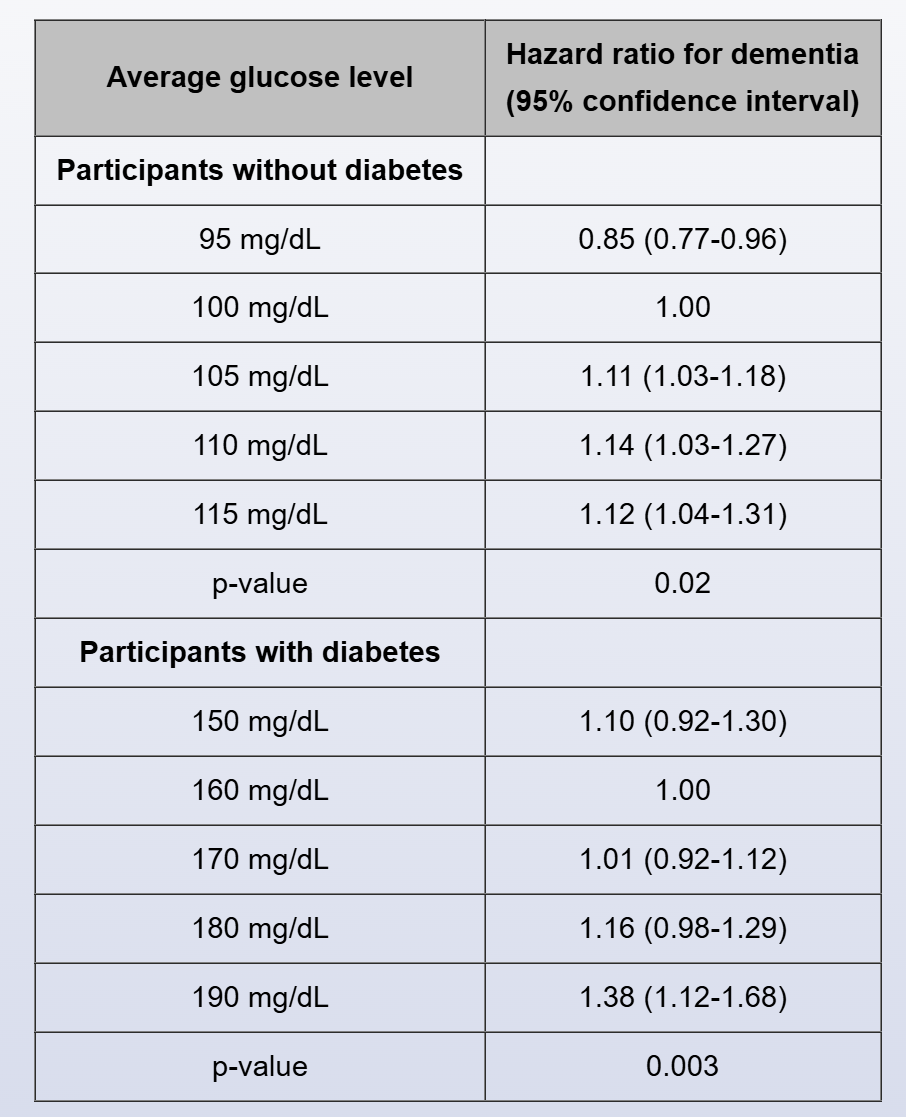
The abstract shown on the left applies to the next 2 items.
A 65-year-old man comes to the clinic for follow-up treatment of hypertension. He is on 2 antihypertensive agents and says he is compliant with his medication regimen. The patient has no other medical problems. Blood pressure is 134/88 mm Hg and pulse is 78/min and regular. BMI is 32 kg/m2. Physical examination is otherwise unremarkable. His fasting blood glucose levels on 2 recent laboratory workups were 108 mg/dL and 111 mg/dL. He is concerned about his risk of dementia because his father suffers from Alzheimer dementia.
Item 1 of 2
Based on the study results, which of the following is the best response to this patient’s concerns?
CorrectIncorrect -
Question 5 of 40
5. Question
Hypothesis: Are higher glucose levels associated with increased risk of dementia?
Methods:
Design: Cohort study.
Setting: Randomly selected members of a single health care system.
Follow-up: Median follow-up of 6.7 years.
Patients: 2,075 participants age ≥65, with diabetes (N = 230) and without diabetes (N = 1,845), all without dementia at baseline.
Exposure variable: Average glucose level for each participant at baseline and in 5-year rolling windows (based on 34,904 clinical measurements of glucose levels and/or 10,518 measurements of glycated hemoglobin levels, with standard transformations according to predetermined formulae applied to arrive at average level for each participant).
Outcome measure: Diagnosis of dementia based on the initial screening with Cognitive Abilities Screening Instrument and confirmed by further evaluation.
Data analysis: Cox regression models stratified according to diabetes status and adjusted for age, sex, study cohort, educational level, level of exercise, blood pressure, and status with respect to coronary and cerebrovascular diseases, atrial fibrillation, smoking, and treatment for hypertension.
Results: During follow-up, dementia developed in 540 participants (79 with diabetes and 461 without). The table reports the adjusted risk of incident dementia associated with the average glucose level over the preceding 5 years among participants without diabetes and those with diabetes.

Item 2 of 2
After excluding from the analysis participants with diabetes who had an unusual clinical course such as high fluctuations in reported glucose levels, the investigators repeat their calculations of the hazard ratio of dementia. This analytical step is best referred to as which of the following?
CorrectIncorrect -
Question 6 of 40
6. Question
A cohort study of 4,000 patients examines the role of vitamin D supplements on the incidence of colon cancer. Relative risk (RR) calculations with their corresponding 95% confidence intervals (CI) are reported for subjects taking vitamin D versus controls after a 5-year follow-up period:
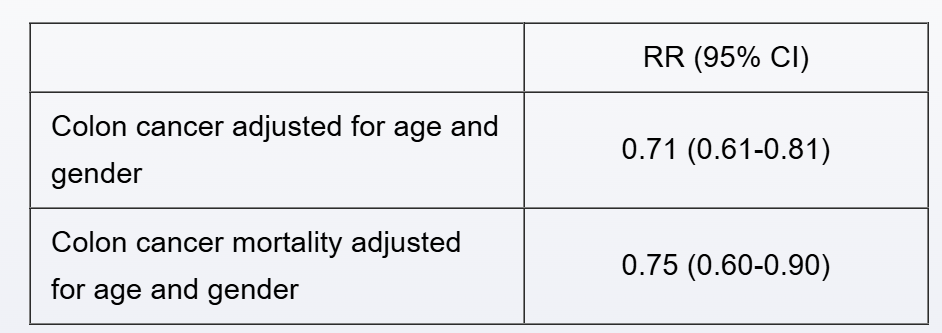
A separate double-blind randomized clinical trial assigns 3,900 subjects to vitamin D supplementation or placebo. The following results are reported for subjects taking vitamin D versus placebo after a 5-year follow-up period:
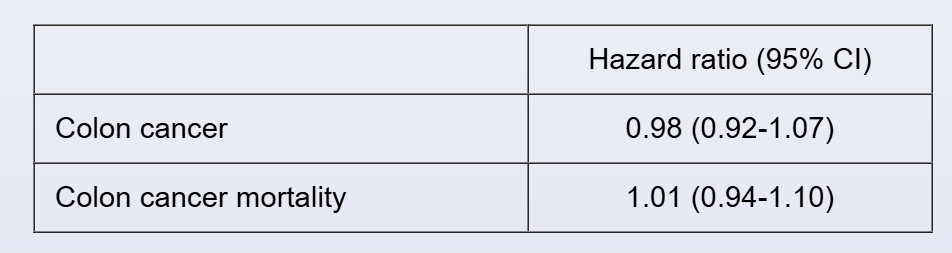
Which of the following best describes the results of both studies?
CorrectIncorrect -
Question 7 of 40
7. Question
A 26-year-old woman comes to the office due to several weeks of fatigue, headaches, joint pain and swelling, light sensitivity, and occasional periods of low-grade fever. The patient has no other medical issues. She works as a systems engineer for a major airline. Family history is unremarkable. The diagnosis of systemic lupus erythematosus (SLE) is considered, and an antinuclear antibody (ANA) assay is obtained. The prevalence of SLE in patients from whom an ANA test is obtained is 3.2%. The sensitivity of ANA for SLE is 96%, and the specificity is 91%. Assuming that this patient has SLE, how likely is she to have a positive ANA result compared with a patient without SLE having a positive ANA result?
CorrectIncorrect -
Question 8 of 40
8. Question
An 11-month-old girl is brought to the pediatrician by her parents for a follow-up visit. The patient was recently diagnosed with a rare multisystemic syndromic disorder characterized by cognitive impairment, deafness, skeletal abnormalities, and coarse facial features. The disorder occurs in ~1 in 100,000 births, and the underlying cause is unknown. The parents ask about factors that may have contributed to the development of the rare disorder. Which of the following types of study designs would be most appropriate to address the parents’ question?
CorrectIncorrect -
Question 9 of 40
9. Question
In a cohort study, 1,800 infertile women with polycystic ovary syndrome (PCOS) who were undergoing their first in vitro fertilization cycle were grouped based on whether they would undergo fresh embryo transfer or embryo cryopreservation followed by frozen embryo transfer. Women underwent the transfer of up to two fresh or two frozen embryos. The primary outcome was a live birth after the first embryo transfer. An excerpt of study results is shown below.
Rate of event
Rate ratio
(95% CI)Outcome
Frozen embryo
transferFresh embryo
transferLive birth after the first transfer
49.3%
42.0%
1.17 (1.05-1.31)
Pregnancy loss
22.0%
32.7%
0.67 (0.54-0.83)
Preeclampsia
4.4%
1.4%
3.12 (1.26-7.73)
Ovarian hyperstimulation syndrome
1.4%
7.1%
0.20 (0.10-0.37)
CI = confidence interval.
There were no significant differences between groups in rates of other pregnancy and neonatal complications. These data best support which of the following conclusions?
CorrectIncorrect -
Question 10 of 40
10. Question
Researchers are designing a study to compare the efficacy and safety of a new selective inhibitor of dipeptidyl peptidase 4 (DPP-4) to placebo in 3,850 patients with type 2 diabetes mellitus (T2DM) and recent acute coronary syndrome. Potential participants will be assigned to receive the new inhibitor or placebo in addition to standard-of-care treatment for T2DM. Throughout the study, patients will also receive treatment for cardiovascular risk factors according to standardized guidelines. The researchers believe that the efficacy of the new inhibitor may be different at different levels of baseline renal function. Based on this information, which of the following is most appropriate to determine whether the researchers’ belief is correct?
CorrectIncorrect -
Question 11 of 40
11. Question
A study examines the role of different biomarkers in the early diagnosis of acute coronary syndrome. The following receiver-operating characteristic (ROC) curves were obtained for different biomarkers upon participants’ initial symptom presentation in the emergency department (the X marks the 99th percentile cutoff point for the corresponding biomarker).
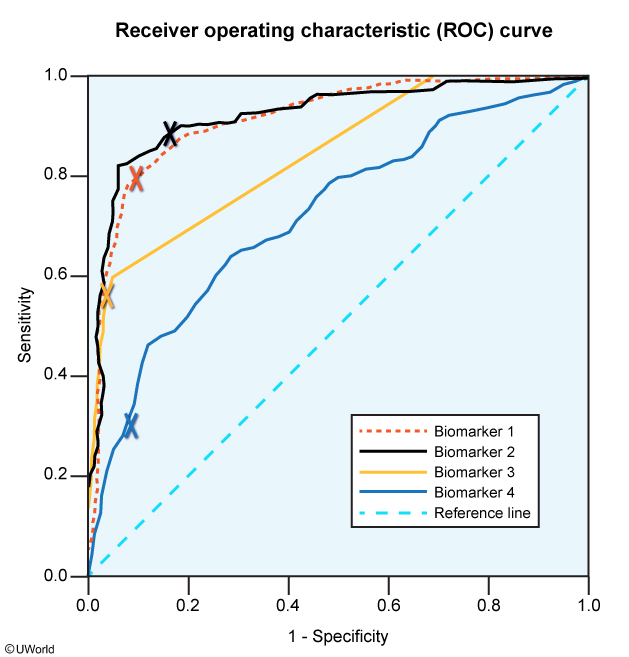
Based on the study results and assuming the differences are statistically significant, which of the following 99th percentile cutoff points can best exclude acute coronary syndrome during initial symptom presentation in the emergency department?
CorrectIncorrect -
Question 12 of 40
12. Question
A group of researchers wants to investigate an outbreak of acute diarrhea that occurred in a coastal town in northern Massachusetts. Approximately 50 people who were from the town or were visiting the surrounding area had severe hemorrhagic diarrhea, in most cases requiring hospitalization for several days. A fatal case was reported. The researchers believe that the outbreak is related to seafood prepared at one of the coastal restaurants. Which of the following study designs is most appropriate to test this hypothesis?
CorrectIncorrect -
Question 13 of 40
13. Question
A randomized, double-blinded clinical trial was conducted to assess the role of a multidrug chemotherapy regimen for the treatment of newly diagnosed stage III and IV stomach cancer. In the study, 150 patients were enrolled in the treatment group and received the new multidrug treatment, and 100 patients were enrolled in the control group and were treated with standard first-line therapy. All patients were followed for 24 months. A total of 120 patients in the treatment group (80%) and 80 patients in the control group (80%) died during this follow-up period. Despite these calculations, the investigators concluded that the new multidrug regimen was more effective than standard therapy. Which of the following most likely accounts for the conclusion reached by the study investigators?
CorrectIncorrect -
Question 14 of 40
14. Question
A study examined the role of biomarkers in diagnosing hypertrophic cardiomyopathy (HCM). The study population included patients without HCM, patients with borderline HCM, and patients with definite HCM. The following echocardiography measurements of the mean left ventricular wall thickness were reported:
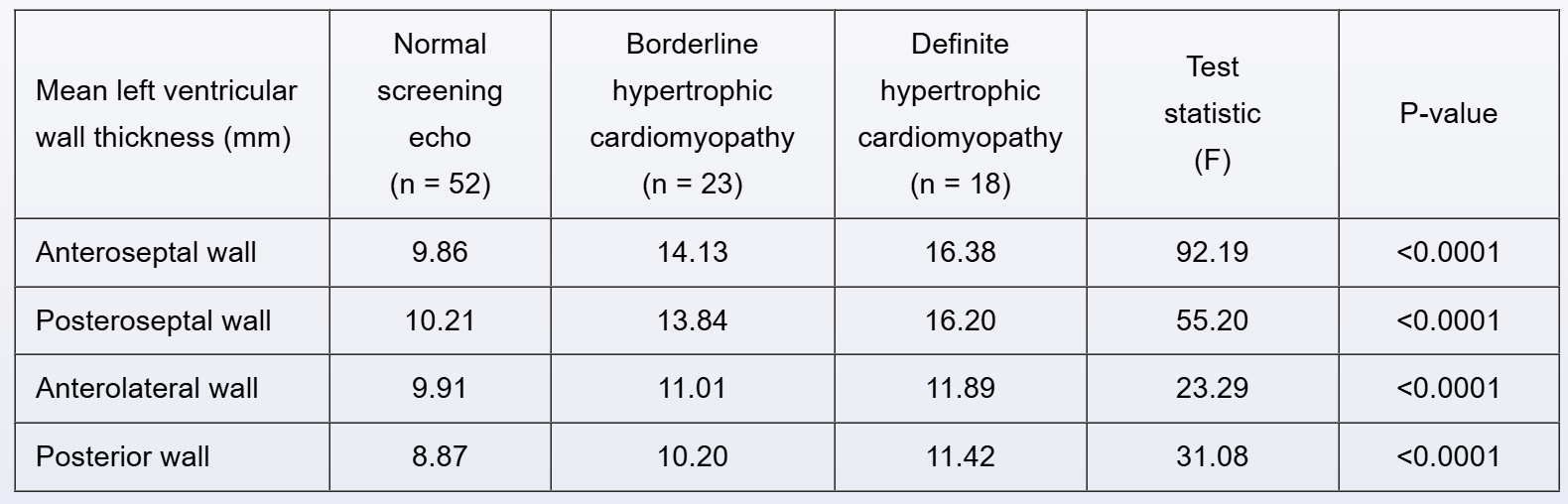
Assuming homoscedasticity and normality, which of the following tests was used to compare measurements?
CorrectIncorrect -
Question 15 of 40
15. Question
Title:
Rivaroxaban in Patients with a Recent Acute Coronary SyndromeHypothesis:
Does inhibition of factor Xa with low-dose rivaroxaban improve cardiovascular outcomes in patients with a recent acute coronary syndrome?Methods:
Design: Randomized clinical trial (Phase 3)Blinding: Double-blind, placebo-controlled trial
Follow-up: Mean duration of treatment with a study drug was 13.1 months.
Setting: Multicenter (766 sites in 44 countries)
Patients: The study included patients (≥ 18 years of age) who had presented with symptoms suggestive of an acute coronary syndrome and in whom an ST segment elevation myocardial infarction (STEMI), a non–ST-segment elevation myocardial infarction (NSTEMI), or unstable angina had been diagnosed.
Intervention: Patients were randomly assigned in a 1:1:1 fashion to twice-daily administration of either 2.5 mg or 5.0 mg of rivaroxaban or placebo.
Outcome measures: The primary efficacy end point was a composite of death from cardiovascular causes, myocardial infarction, or stroke. The primary safety end point was TIMI (Thrombolysis In Myocardial Infarction) bleeding not related to coronary-artery bypass grafting (CABG).
Results:
A total of 15,526 patients underwent randomization. Patients were randomly assigned to receive 2.5 mg of rivaroxaban (5174 patients), 5.0 mg of rivaroxaban (5176), or placebo (5176). Hazard ratios (HR) and their associated 95% confidence intervals are listed below.Rivaroxaban 2.5 mg Twice Daily vs. Placebo
Rivaroxaban 5 mg Twice Daily vs. Placebo
Rivaroxaban Combined vs. Placebo
Primary efficacy end point of death from cardiovascular causes, myocardial infarction, or stroke
0.84
(0.72–0.97)0.85
(0.73–0.98)0.84
(0.74 to 0.96)Death from cardiovascular causes
0.66
(0.51–0.86)0.94
(0.75–1.20)0.80
(0.65–0.99)Death from myocardial infarction
0.90
(0.75–1.09)0.79
(0.65–0.97)0.85
(0.72–1.00)Death from Stroke
1.13
(0.74–1.73)1.34
(0.90–2.02)1.24
(0.86–1.78)Death from any cause, myocardial infaction, or stroke – secondary end point
0.83
(0.72 – 0.97)0.84
(0.73 – 0.98)0.84
(0.74 – 0.95)TIMI major bleeding not associated with CABG
3.46
(2.08–5.77)4.47
(2.71–7.36)3.96
(2.46–6.38)TIMI minor bleeding
1.62
(0.92–2.82)2.52
(1.50–4.24)2.07
(1.27–3.37)TIMI bleeding requiring medical attention
1.79
(1.55–2.07)2.39
(2.08–2.75)2.09
(1.83–2.38)Intracranial hemorrhage
2.83
(1.02–7.86)3.74
(1.39–10.07)3.28
(1.28–8.42)Fatal bleeding
0.67
(0.24–1.89)1.72
(0.75–3.92)1.19
(0.54–2.59)Conclusion:
In patients with a recent acute coronary syndrome, rivaroxaban reduced the risk of the composite end point of death from cardiovascular causes, myocardial infarction, or stroke.Funding Source: Funded by Johnson & Johnson and Bayer Healthcare; ATLAS ACS 2–TIMI 51 ClinicalTrials.gov number, NCT00809965)
Structured abstract is based on: N Engl J Med. 2011 Nov 13. Epub ahead of print.
The abstract shown on the left applies to the next 3 items
Item 1 of 3
A 62-year-old man with a history of dyslipidemia and type 2 diabetes mellitus comes to the emergency department due to severe substernal chest pain and diaphoresis that began 2 hours ago. His ECG shows ST- segment elevation in leads V1 to V4. He is taken to the cardiac catheterization lab and a complete occlusion of the mid-left anterior descending artery (LAD) is diagnosed. A drug-eluting stent is placed in the LAD with complete restoration of flow, and the patient is started on aspirin and clopidogrel. On the third day of hospitalization, the option of adding rivaroxaban is discussed with the patient. He asks about the benefits and risks of the added therapy. Based on the research abstract, which of the following measures would best reflect the net clinical benefit of rivoraxaban?
CorrectIncorrect -
Question 16 of 40
16. Question
Title:
Rivaroxaban in Patients with a Recent Acute Coronary SyndromeHypothesis:
Does inhibition of factor Xa with low-dose rivaroxaban improve cardiovascular outcomes in patients with a recent acute coronary syndrome?Methods:
Design: Randomized clinical trial (Phase 3)Blinding: Double-blind, placebo-controlled trial
Follow-up: Mean duration of treatment with a study drug was 13.1 months.
Setting: Multicenter (766 sites in 44 countries)
Patients: The study included patients (≥ 18 years of age) who had presented with symptoms suggestive of an acute coronary syndrome and in whom an ST segment elevation myocardial infarction (STEMI), a non–ST-segment elevation myocardial infarction (NSTEMI), or unstable angina had been diagnosed.
Intervention: Patients were randomly assigned in a 1:1:1 fashion to twice-daily administration of either 2.5 mg or 5.0 mg of rivaroxaban or placebo.
Outcome measures: The primary efficacy end point was a composite of death from cardiovascular causes, myocardial infarction, or stroke. The primary safety end point was TIMI (Thrombolysis In Myocardial Infarction) bleeding not related to coronary-artery bypass grafting (CABG).
Results:
A total of 15,526 patients underwent randomization. Patients were randomly assigned to receive 2.5 mg of rivaroxaban (5174 patients), 5.0 mg of rivaroxaban (5176), or placebo (5176). Hazard ratios (HR) and their associated 95% confidence intervals are listed below.Rivaroxaban 2.5 mg Twice Daily vs. Placebo
Rivaroxaban 5 mg Twice Daily vs. Placebo
Rivaroxaban Combined vs. Placebo
Primary efficacy end point of death from cardiovascular causes, myocardial infarction, or stroke
0.84
(0.72–0.97)0.85
(0.73–0.98)0.84
(0.74 to 0.96)Death from cardiovascular causes
0.66
(0.51–0.86)0.94
(0.75–1.20)0.80
(0.65–0.99)Death from myocardial infarction
0.90
(0.75–1.09)0.79
(0.65–0.97)0.85
(0.72–1.00)Death from Stroke
1.13
(0.74–1.73)1.34
(0.90–2.02)1.24
(0.86–1.78)Death from any cause, myocardial infaction, or stroke – secondary end point
0.83
(0.72 – 0.97)0.84
(0.73 – 0.98)0.84
(0.74 – 0.95)TIMI major bleeding not associated with CABG
3.46
(2.08–5.77)4.47
(2.71–7.36)3.96
(2.46–6.38)TIMI minor bleeding
1.62
(0.92–2.82)2.52
(1.50–4.24)2.07
(1.27–3.37)TIMI bleeding requiring medical attention
1.79
(1.55–2.07)2.39
(2.08–2.75)2.09
(1.83–2.38)Intracranial hemorrhage
2.83
(1.02–7.86)3.74
(1.39–10.07)3.28
(1.28–8.42)Fatal bleeding
0.67
(0.24–1.89)1.72
(0.75–3.92)1.19
(0.54–2.59)Conclusion:
In patients with a recent acute coronary syndrome, rivaroxaban reduced the risk of the composite end point of death from cardiovascular causes, myocardial infarction, or stroke.Funding Source: Funded by Johnson & Johnson and Bayer Healthcare; ATLAS ACS 2–TIMI 51 ClinicalTrials.gov number, NCT00809965)
Structured abstract is based on: N Engl J Med. 2011 Nov 13. Epub ahead of print.
Item 2 of 3
The statistical methods section of the full research paper indicates that the analysis was performed using an “intention-to-treat” approach. The primary purpose of such an approach is most likely which of the following?
CorrectIncorrect -
Question 17 of 40
17. Question
Title:
Rivaroxaban in Patients with a Recent Acute Coronary SyndromeHypothesis:
Does inhibition of factor Xa with low-dose rivaroxaban improve cardiovascular outcomes in patients with a recent acute coronary syndrome?Methods:
Design: Randomized clinical trial (Phase 3)Blinding: Double-blind, placebo-controlled trial
Follow-up: Mean duration of treatment with a study drug was 13.1 months.
Setting: Multicenter (766 sites in 44 countries)
Patients: The study included patients (≥ 18 years of age) who had presented with symptoms suggestive of an acute coronary syndrome and in whom an ST segment elevation myocardial infarction (STEMI), a non–ST-segment elevation myocardial infarction (NSTEMI), or unstable angina had been diagnosed.
Intervention: Patients were randomly assigned in a 1:1:1 fashion to twice-daily administration of either 2.5 mg or 5.0 mg of rivaroxaban or placebo.
Outcome measures: The primary efficacy end point was a composite of death from cardiovascular causes, myocardial infarction, or stroke. The primary safety end point was TIMI (Thrombolysis In Myocardial Infarction) bleeding not related to coronary-artery bypass grafting (CABG).
Results:
A total of 15,526 patients underwent randomization. Patients were randomly assigned to receive 2.5 mg of rivaroxaban (5174 patients), 5.0 mg of rivaroxaban (5176), or placebo (5176). Hazard ratios (HR) and their associated 95% confidence intervals are listed below.Rivaroxaban 2.5 mg Twice Daily vs. Placebo
Rivaroxaban 5 mg Twice Daily vs. Placebo
Rivaroxaban Combined vs. Placebo
Primary efficacy end point of death from cardiovascular causes, myocardial infarction, or stroke
0.84
(0.72–0.97)0.85
(0.73–0.98)0.84
(0.74 to 0.96)Death from cardiovascular causes
0.66
(0.51–0.86)0.94
(0.75–1.20)0.80
(0.65–0.99)Death from myocardial infarction
0.90
(0.75–1.09)0.79
(0.65–0.97)0.85
(0.72–1.00)Death from Stroke
1.13
(0.74–1.73)1.34
(0.90–2.02)1.24
(0.86–1.78)Death from any cause, myocardial infaction, or stroke – secondary end point
0.83
(0.72 – 0.97)0.84
(0.73 – 0.98)0.84
(0.74 – 0.95)TIMI major bleeding not associated with CABG
3.46
(2.08–5.77)4.47
(2.71–7.36)3.96
(2.46–6.38)TIMI minor bleeding
1.62
(0.92–2.82)2.52
(1.50–4.24)2.07
(1.27–3.37)TIMI bleeding requiring medical attention
1.79
(1.55–2.07)2.39
(2.08–2.75)2.09
(1.83–2.38)Intracranial hemorrhage
2.83
(1.02–7.86)3.74
(1.39–10.07)3.28
(1.28–8.42)Fatal bleeding
0.67
(0.24–1.89)1.72
(0.75–3.92)1.19
(0.54–2.59)Conclusion:
In patients with a recent acute coronary syndrome, rivaroxaban reduced the risk of the composite end point of death from cardiovascular causes, myocardial infarction, or stroke.Funding Source: Funded by Johnson & Johnson and Bayer Healthcare; ATLAS ACS 2–TIMI 51 ClinicalTrials.gov number, NCT00809965)
Structured abstract is based on: N Engl J Med. 2011 Nov 13. Epub ahead of print.
Item 3 of 3
After a pharmaceutical drug is approved by the Food and Drug Administration, phase IV or post-marketing surveillance is conducted to detect fatal and serious side effects. The failure to detect such events in earlier phases of clinical testing is most likely due to which of the following?
CorrectIncorrect -
Question 18 of 40
18. Question
A 35-year-old man comes to the emergency department due to 2 days of fever and dysuria. He has a history of paraplegia as the result of a gunshot wound 5 years ago that required surgical intervention. He takes narcotics for chronic pain that has developed since the surgery. The patient is bedbound and practices intermittent urinary self-catheterization. He has had multiple admissions for recurrent urinary tract infections; urine cultures have grown multidrug-resistant organisms requiring intravenous antibiotic therapy in the past. He most recently completed a course of antibiotics a week ago. The patient says, “It feels like my urinary tract infection is coming back—I was just done with antibiotics and was hoping I would get a break!” He reports that every 5 years of life in his current state of health are equivalent to 1 year of life in full health. Which of the following measures is best described by this value?
CorrectIncorrect -
Question 19 of 40
19. Question
A study is conducted to assess myocardial perfusion imaging performance using a new pharmacologic agent for stress testing. The test is performed on inpatients and outpatients with chest pain. Patients with positive stress test results underwent cardiac catheterization to diagnose flow-limiting coronary artery disease (CAD). Physicians interpreting the catheterization radiographs were blinded to inpatient/outpatient status. The following results are obtained:
Inpatients:
Number of patients with positive stress test: 122
Flow-limiting CAD on cardiac catheterization: 102/122 (84%)Outpatients:
Number of patients with positive stress test: 115
Flow-limiting CAD on cardiac catheterization: 67/115 (58%)Which of the following best explains the difference in the rate of flow-limiting CAD between the groups in this study?
CorrectIncorrect -
Question 20 of 40
20. Question
An open-label randomized trial is conducted to compare a new nonsteroidal anti-inflammatory agent (AG) and low-dose colchicine for the treatment of gout flares in primary care. A total of 400 adults with gout flares were randomized 1:1 to AG for 7 days or low-dose colchicine three times per day for 4 days. The primary outcome was change in pain intensity from baseline measured over the first 7 days. At Day 7 follow-up, about 25% of participants in the low-dose colchicine group had switched treatment to AG, and about 15% of participants in the low-dose colchicine group had stopped treatment altogether. The primary investigator decided to preserve the original randomization for the data analysis. Which of the following describes the data analysis approach adopted by the primary investigator?
CorrectIncorrect -
Question 21 of 40
21. Question
Researchers are interested in further investigating the side effects of a relatively recent hypolipidemic drug. A literature review showed several studies in which patients who took this drug reported severe acute myositis as an adverse event. In many instances, affected patients experienced life-threatening complications from myositis, including rhabdomyolysis leading to acute renal failure and profound electrolyte abnormalities. However, none of these studies documented a statistically significant difference in the occurrence of severe acute myositis between the treatment and placebo groups. Which of the following is the best method to further investigate the association between use of this hypolipidemic drug and development of severe acute myositis?
CorrectIncorrect -
Question 22 of 40
22. Question
A group of researchers is designing a study to investigate the role of a new diet in treating patients with mild hypertension. The researchers are using the following parameters to calculate the sample size required for the study:
-
Expected change (decrease) in systolic blood pressure (SBP) with the new diet: 8 mm Hg
-
Expected standard deviation for SBP in the population: 10 mm Hg
-
Value for α: 0.01
-
Power: 0.85
Based on these parameters, they estimate the minimum sample size required for a 2-tailed test to be 21. In which of the following situations would a sample size >21 be needed to appropriately address the research question?
CorrectIncorrect -
-
Question 23 of 40
23. Question
Abstract:
Title: Low-level environmental lead exposure in childhood and adult intellectual function.
Hypothesis: Lead exposure in childhood predicts intellectual functioning in young adulthood.
Methods:
-
Design: Retrospective cohort study.
-
Participants: From an initial cohort of 249 infants established in 1974, 148 (59.4%) were still being followed at year 10. A total of 43 of the 148 (29%) young adults (mean age: 29) completed the study. These 43 participants were similar to the other members of the original cohort (who did not participate or undergo neuropsychological assessment) in terms of demographic factors, measures of socioeconomic status, blood-lead history, and intellectual function (IQ) scores in early childhood.
-
Measures of exposure: Exposure to lead measured as blood-lead levels from samples obtained at birth and at age 6 months, 10 months, 12 months, 2 years, 4 years, and 10 years.
-
Outcome measures: IQ of subjects was measured using the Wechsler Abbreviated Scale of Intelligence (WASI). A child neurologist, trained in the administration of the instrument and unaware of the subject’s developmental history and lead concentrations, administered the WASI to all subjects.
-
Covariates: All analyses included prespecified covariates, which consisted of established predictors of children’s intellectual outcomes, factors widely used in studies of lead exposure and intelligence, and factors in early adulthood that have been shown to affect intellectual outcome.
Results: Childhood blood-lead concentration, defined as the mean of the blood-lead concentrations at ages 4 and 10 years, had the strongest relationship with IQ (p = 0.01). IQ was also significantly related to blood-lead concentration at age 6 months (p = 0.03), 4 years (p = 0.03), and 10 years (p = 0.02). After adjusting for maternal IQ, the association was still evident; however, due to the small sample size, the significance of the association was altered (−1.89 with a 95% confidence interval [95%CI] [−2.40, −0.47] before adjusting for maternal IQ to −1.81 with a 95%CI [−2.59, 0.06] after adjusting for maternal IQ).
Conclusions: The study suggests that lead exposure in childhood predicts intellectual functioning in young adulthood. Given the small sample size, however, the potentially confounding effects of maternal IQ cannot be excluded.
The abstract shown applies to the next 2 items.
A team of neurologists and psychologists researching intellectual function is reviewing the results of a study on the association between childhood lead exposure and adult intellectual function.
Item 1 of 2
Based on the abstract, which of the following is the most concerning factor regarding the design of the study?
CorrectIncorrect -
-
Question 24 of 40
24. Question
Abstract:
Title: Low-level environmental lead exposure in childhood and adult intellectual function.
Hypothesis: Lead exposure in childhood predicts intellectual functioning in young adulthood.
Methods:
-
Design: Retrospective cohort study.
-
Participants: From an initial cohort of 249 infants established in 1974, 148 (59.4%) were still being followed at year 10. A total of 43 of the 148 (29%) young adults (mean age: 29) completed the study. These 43 participants were similar to the other members of the original cohort (who did not participate or undergo neuropsychological assessment) in terms of demographic factors, measures of socioeconomic status, blood-lead history, and intellectual function (IQ) scores in early childhood.
-
Measures of exposure: Exposure to lead measured as blood-lead levels from samples obtained at birth and at age 6 months, 10 months, 12 months, 2 years, 4 years, and 10 years.
-
Outcome measures: IQ of subjects was measured using the Wechsler Abbreviated Scale of Intelligence (WASI). A child neurologist, trained in the administration of the instrument and unaware of the subject’s developmental history and lead concentrations, administered the WASI to all subjects.
-
Covariates: All analyses included prespecified covariates, which consisted of established predictors of children’s intellectual outcomes, factors widely used in studies of lead exposure and intelligence, and factors in early adulthood that have been shown to affect intellectual outcome.
Results: Childhood blood-lead concentration, defined as the mean of the blood-lead concentrations at ages 4 and 10 years, had the strongest relationship with IQ (p = 0.01). IQ was also significantly related to blood-lead concentration at age 6 months (p = 0.03), 4 years (p = 0.03), and 10 years (p = 0.02). After adjusting for maternal IQ, the association was still evident; however, due to the small sample size, the significance of the association was altered (−1.89 with a 95% confidence interval [95%CI] [−2.40, −0.47] before adjusting for maternal IQ to −1.81 with a 95%CI [−2.59, 0.06] after adjusting for maternal IQ).
Conclusions: The study suggests that lead exposure in childhood predicts intellectual functioning in young adulthood. Given the small sample size, however, the potentially confounding effects of maternal IQ cannot be excluded.
Item 2 of 2
Which of the following is the best approach to further investigate the association between environmental lead exposure in childhood and adult intellectual function?
CorrectIncorrect -
-
Question 25 of 40
25. Question
A randomized, double-blind clinical trial is conducted to evaluate the effectiveness of Drug X for the control of mild hypertension in adults. A total of 450 patients age ≥18 with mild hypertension are randomly assigned (ratio: 1:1:1) to 20 mg of Drug X (Dose A), 50 mg of Drug X (Dose B), or a standard dose of an established antihypertensive drug (Control). Patients are followed over a 12-week period and the changes in blood pressure, laboratory test results, and endothelium function are compared between treatment groups. Researchers are concerned that an unequal distribution of BMI between treatment groups may affect the results of the study. The graph below shows the distribution of BMI categories in each treatment group.
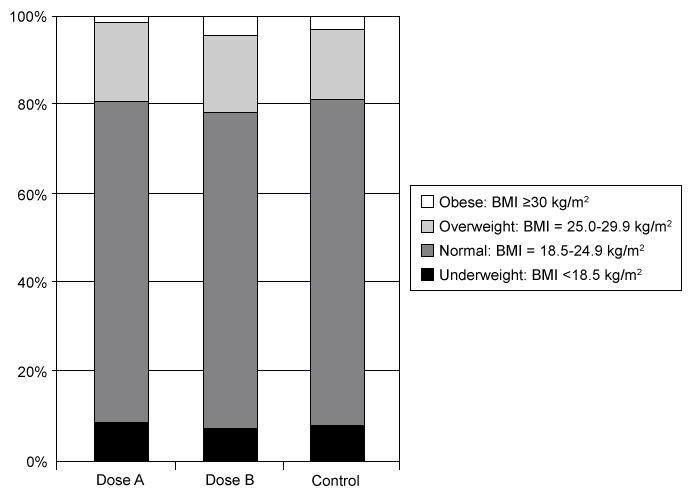
A chi-square test is conducted to address their concern and yields a p-value of 0.03. If a significance level of 0.01 is considered, which of the following statements is true?
CorrectIncorrect -
Question 26 of 40
26. Question
A pharmaceutical company plans to conduct a study to evaluate the efficacy of the addition of a novel conjugated anti-CD22 monoclonal antibody (mAb) to frontline therapy in adults age 18-39 with newly diagnosed precursor B-cell acute lymphoblastic leukemia (ALL). Researchers will assess the adverse reactions associated with treatment with the anti-CD22 mAb. A total of 1,200 patients newly diagnosed with CD22 positive B-cell ALL will be randomized to either frontline chemotherapy or intravenous infusion of the mAb given on days 1, 8, and 15. All participants will undergo bone marrow aspirate and biopsy on day 28. The study’s primary outcome measure will be event-free survival, and its secondary outcome will include disease-free survival, overall survival, and complete response. Which of the following best describes this type of study?
CorrectIncorrect -
Question 27 of 40
27. Question
The drug ad applies to the next 2 items
The drug advertisement suggests the addition of Zettiga (extended-release niacin) to low-dose simvastatin for the treatment of high-risk patients with type 2 hyperlipidemia or mixed dyslipidemia.
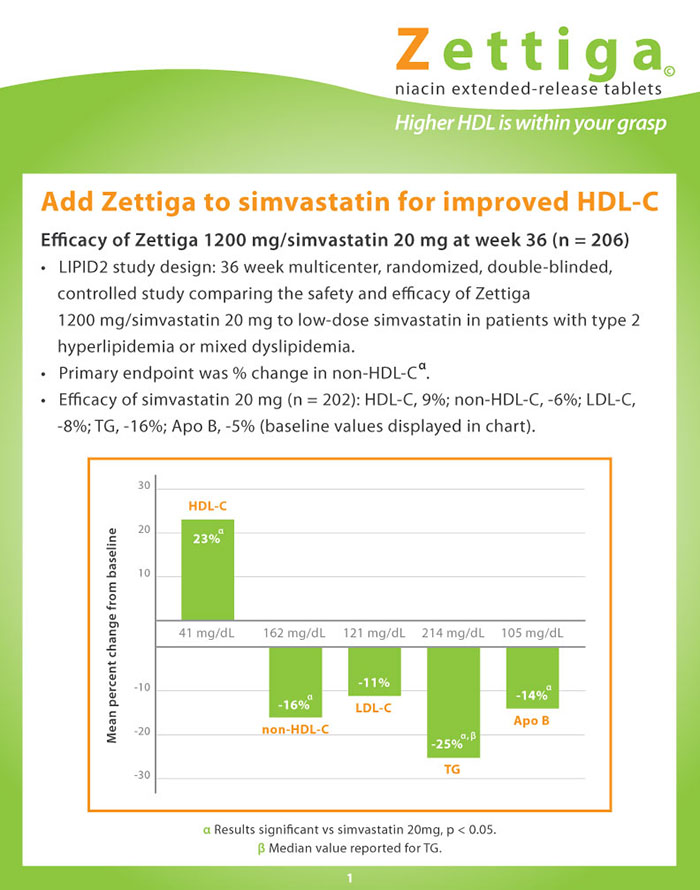

Item 1 of 2
A 56-year-old man with exertional chest pain comes to the office because his exercise stress echocardiogram showed evidence of mild ischemia in the posterior wall. He was recently diagnosed with type 2 diabetes mellitus and hypertension. He is a current smoker but is willing to quit. His father died of a heart attack at age 51. Physical examination is unremarkable.
Laboratory results are as follows:
Creatinine
1.1 mg/dL
High-density lipoprotein
41 mg/dL
Low-density lipoprotein
122 mg/dL
Triglycerides
220 mg/dL
HemoglobinA1c
7.6%
Based on the information in the drug advertisement, which of the following changes in HDL and LDL cholesterol would be expected if this patient was started on combined Zettiga and simvastatin compared to simvastatin alone?
 CorrectIncorrect
CorrectIncorrect -
Question 28 of 40
28. Question
The drug ad applies to the next 2 items
The drug advertisement suggests the addition of Zettiga (extended-release niacin) to low-dose simvastatin for the treatment of high-risk patients with type 2 hyperlipidemia or mixed dyslipidemia.


Item 2 of 2
According to the drug advertisement, the investigators reported the median value and the median percentage change from baseline for triglyceride levels in the study patients. Which of the following best explains the reason for reporting median values as opposed to mean values?
CorrectIncorrect -
Question 29 of 40
29. Question
Researchers are conducting a clinical trial to assess the effect of erythropoietin on the survival of patients with end-stage renal disease receiving dialysis. Statistical analysis of the data shows that, after adjustment for age, sex, and duration of disease, patients who did not receive erythropoietin were at increased risk of mortality compared with those who did; the calculated relative risk (RR) of survival and corresponding p-value for patients treated with erythropoietin were RR = 2.4 with p = 0.005. Among patients with diabetes, erythropoietin use was associated with higher survival rates, with an RR of 6.2 and p < 0.001. Among patients without diabetes, however, the corresponding RR was 0.94 with p = 0.56. The researchers are preparing a manuscript for publication of their findings. Which of the following recommendations is most appropriate for the manuscript?
CorrectIncorrect -
Question 30 of 40
30. Question
The Health Belief Model (HBM) construct is used to determine why individuals follow disease-preventing behaviors. One of its components is self-efficacy, referring to an individual’s confidence in the ability to successfully perform a disease-preventing behavior. A randomized controlled trial investigates the efficacy of an HBM-based intervention incorporating self-efficacy to empower pregnant women to reduce secondhand smoke (SHS) exposure. A group of 150 nonsmoking pregnant women age ≥18 is randomized into 3 groups: group-based HBM intervention, individual-based HBM intervention, or treatment-as-usual (control). A questionnaire is administered at baseline and at 2-month follow-up with the goal of comparing health beliefs, self-efficacy for rejecting SHS exposure, and SHS rejection behaviors between the 3 groups at baseline and at 2-month follow-up. The outcomes are measured using standard quantitative HBM construct scores. An excerpt of study results is shown below.
Health beliefs, self-efficacy & behaviors of rejecting SHS* after intervention
2-months follow-up
Variable
Group-based intervention
Individual-based intervention
Treatment-as-usual (control)
Health beliefs Mean
SD
Mean
SD
Mean
SD
p-value Perceived SHS-related disease susceptibility 64.78
4.13
61.00
4.78
59.75
5.02
<0.0001
Perceived benefits of rejecting SHS exposure 44.90
3.71
42.60
3.75
43.12
3.29
<0.0001
Self-efficacy for rejecting SHS exposure 38.26
3.24
34.10
5.21
33.50
4.02
<0.0001
SHS rejection behaviors 87.78
6.77
85.00
6.88
77.92
8.32
<0.0001
*Variables measured using standard quantitative HBM construct scores. HBM = Health Belief Model; SD = standard deviation; SHS = secondhand smoke.
Assuming all conditions for statistical inference are met, which of the following tests was used to compare outcomes?
CorrectIncorrect -
Question 31 of 40
31. Question
A group of clinical researchers discovered a new ovarian cancer biomarker that is suitable for early disease diagnosis and prognosis, and that may ultimately lead to improved patient management and outcomes. The researchers measured the new serum biomarker in 850 women, 350 of whom had been recently diagnosed with ovarian cancer and 500 of whom had no history of ovarian cancer. The data analysis included a stratified analysis based on levels of the serum biomarker. An excerpt of the results is shown below.
Concentration of new biomarker for
ovarian cancerNumber of women positive for
ovarian cancerNumber of women negative for
ovarian cancer0-2.5 μg/L
35
255
2.6-4.5 μg/L
52
185
4.6-6.5 μg/L
123
40
≥6.6 μg/L
140
20
A researcher is interested in the likelihood ratio associated with a serum biomarker concentration ≥6.6 μg/L. Which of the following is closest to the value the researcher should report?
CorrectIncorrect -
Question 32 of 40
32. Question
A large study evaluated a rapid toxin-based stool assay for diagnosing Clostridium difficile colitis. It evaluated the test against polymerase chain reaction, which served as the gold standard. The following values of the new test were obtained from a hospitalized symptomatic patient population with 50% prevalence of Clostridium difficile colitis:
Sensitivity
80%
Specificity
90%
Positive predictive value
89%
Negative predictive value
82%
Which of the following is the positive likelihood ratio for the new test?
CorrectIncorrect -
Question 33 of 40
33. Question
Researchers are conducting a cross-sectional study to demonstrate the relationship between renal frame count (RFC), considered the dependent variable, and LDL, considered an independent variable. RFC is an objective quantitative angiographic method of measuring macrovascular blood flow in the main renal artery and its segmental branches. Two hundred patients were categorized into 2 groups according to serum LDL levels—LDL <130 mg/dL (n = 90) and LDL ≥130 mg/dL (n = 110). Other parameters included BMI, platelet count, and creatinine clearance. Which of the following is the best statistical approach for simultaneously evaluating the association between RFC and LDL cholesterol while adjusting for BMI, platelet count, and creatinine clearance?
CorrectIncorrect -
Question 34 of 40
34. Question
An orthotics and prosthetics company conducts a single-blind, randomized, parallel group trial to investigate the effects of custom foot orthoses with motion control shoes versus the effects of motion control shoes alone in individuals with patellofemoral joint osteoarthritis. Eligible subjects are randomized to receive either custom foot orthoses with motion control shoes or motion control shoes alone, to be worn for 4 months. A significant number of patients are lost to follow-up or are noncompliant to the assigned intervention during the study. However, results for the patients who completed the protocol are encouraging. Which of the following techniques is most appropriate to estimate the real effect of custom foot orthoses in the population?
CorrectIncorrect -
Question 35 of 40
35. Question
A study examines the relationship between urine sodium excretion and systolic blood pressure in a large cohort of patients from several countries. The following results are reported for change in systolic blood pressure for every 1-g increase in urine sodium excretion:
N
Change in systolic blood pressure in mm Hg for every 1-g increase in urine sodium excretion
(95% confidence interval)P-value for interaction
Total
98,029
2.01 (2.00–2.31)
Sodium excretion
<0.001
<3 g/day
11,132
0.68 (-0.32–1.92)
3–5 g/day
44,143
1.80 (1.19–2.09)
>5 g/day
42,754
2.71 (2.37–2.81)
Hypertensive state
<0.001
No hypertension
55,271
1.38 (1.24–1.41)
Hypertension
42,758
2.37 (2.31–2.71)
Age
<0.001
<45 years
30,910
2.01 (1.91–2.21)
45–55 years
32,217
2.48 (2.26–2.71)
>55 years
34,902
3.01 (2.80–3.23)
Which of the following best describes the study results?
CorrectIncorrect -
Question 36 of 40
36. Question
Researchers are evaluating a diagnostic test for Legionella infection that relies on detection of bacterial antigens in the serum. The test is compared to bacterial cultures, which are the gold standard for diagnosis. A sample of 200 patients is selected. Among 100 patients with culture-positive infection, the antigen test was positive in 90 patients. Among 100 patients with negative cultures, the test was positive in 30 patients. What was the positive predictive value of the new serum antigen test in this sample?
CorrectIncorrect -
Question 37 of 40
37. Question
A 24-year-old man comes to the emergency department due to persistent palpitations for the past 4 hours. The patient has never experienced palpitations previously. He has no medical history and takes no medications. ECG shows a regular, narrow-complex tachycardia (QRS duration of 90 msec) at a rate of 205/min, suggestive of atrioventricular reentry tachycardia. Blood pressure is 135/80 mm Hg. Physical examination is notable for tachycardia but is otherwise unremarkable. Vagal maneuvers are ineffective in terminating the tachycardia. The patient then receives a rapid intravenous push of adenosine (6 mg) followed by a saline bolus, which results in a brief return of sinus rhythm immediately followed by an irregular, wide-complex tachycardia at 230/min. This rhythm degenerates into ventricular fibrillation, and the patient loses consciousness. He is immediately defibrillated and regains consciousness. Repeat ECG shows normal sinus rhythm at a rate of 89/min, short PR interval of 110 msec, prolonged QRS complex of 130 msec, and a delta wave in multiple leads. Which of the following best classifies this patient’s encounter?
CorrectIncorrect -
Question 38 of 40
38. Question
A study examines participation in cardiac rehabilitation in patients following their first-ever episode of myocardial infarction in Oakland County, Michigan, from 1991-2010. The following graphs are reported:
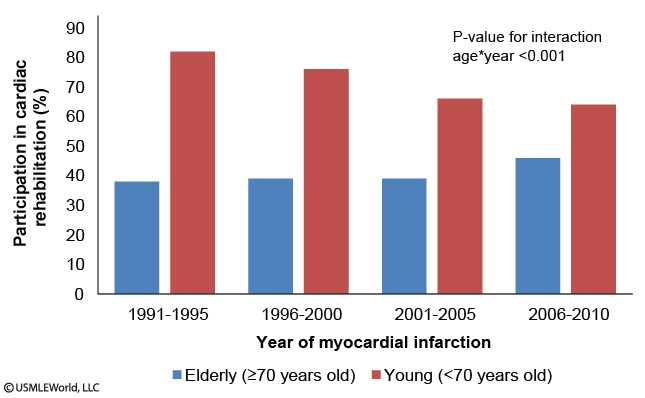
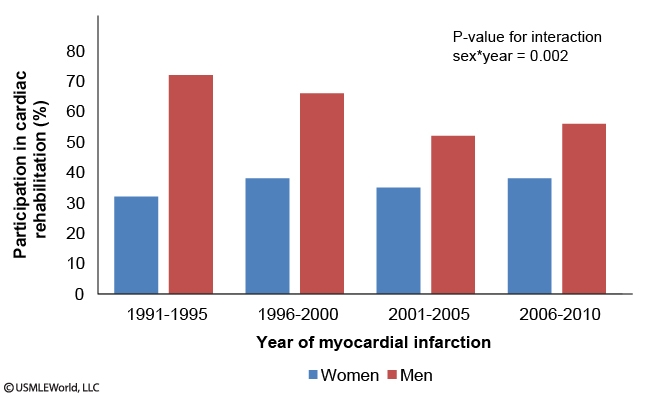
Which of the following is the best statement based on the information presented?
CorrectIncorrect -
Question 39 of 40
39. Question
A randomized study assigned patients with sickle cell anemia and silent stroke to either monthly transfusion therapy (transfusion group of 110 patients) or usual care (observation group of 105 patients). An excerpt of the study results is provided below:
Adverse event
N
Total adverse events
Incidence rate ratio
(95% confidence interval)P-value
Acute chest syndrome
0.14 (0.05–0.31)
<0.001
Observation group
105
43
Transfusion group
110
7
Avascular hip necrosis (symptomatic)
0.27 (0.04–0.81)
0.03
Observation group
105
7
Transfusion group
110
2
Headache
0.69 (0.32–1.21)
0.51
Observation group
105
89
Transfusion group
110
79
Iron overload (ferritin >1500 ng/mL)
16.41 (6.21–849.21)
<0.001
Observation group
35
38
Transfusion group
91
1370
Vasoocclusive crisis
0.42 (0.18–0.79)
0.004
Observation group
105
301
Transfusion group
110
143
Which of the following adverse event estimates is most subject to selection bias?
CorrectIncorrect -
Question 40 of 40
40. Question
A randomized controlled trial investigated the effect of body awareness therapy (BAT) and aerobic exercises on pain and quality of life in patients with tension-type headache. Participants were randomly assigned to 1 of 3 groups: BAT group, aerobic exercise (AE) group, and control group. Main outcomes were pain severity evaluated by a Visual Analog Scale (VAS); pain-related disability measured by the Pain Disability Index (PDI); impact of headaches measured by the Headache Impact Tests (HIT); and quality of life measured by the Short Form Health Survey (SF-36). Baseline characteristics concerning age, weight, height, BMI, VAS, and HIT were not statistically different among the groups (p > 0.05). However, there was a statistically significant difference among the groups (p < 0.05) in mean baseline PDI values. Further evaluation of baseline PDI values revealed the following:
Outcome
Group
Baseline values
Mean
Median
Mode
Pain disability index
Aerobic exercise
33.27
31.48
30.94
Body awareness therapy
43.85
30.41
27.53
Control
28.71
30.85
31.01
Which of the following is likely to be the most valid measure of central tendency for baseline PDI data?
CorrectIncorrect
